The Gatekeeper’s Fault Line: Deconstructing the George Tidmarsh Resignation and the Crisis of Trust at the FDA
An intelligence briefing on the structural fissures compromising America’s public health apparatus and what it signals for investors, policymakers, and the public.
The abrupt resignation of Dr. George Tidmarsh, Director of the FDA’s Center for Drug Evaluation and Research (CDER), on November 2, 2025, is far more than a personnel change; it is a seismic event exposing deep, structural fault lines within America’s foremost public health institution. Occurring amidst a federal probe into his conduct and a lawsuit from a pharmaceutical company, Tidmarsh’s departure is a critical inflection point. It forces a confrontation with uncomfortable truths about the FDA’s vulnerability to internal conflicts, industry pressure, and the erosion of the public’s confidence.
For industry leaders, investors, and policymakers, this is not a spectator sport. The shockwaves from this event will redefine regulatory risk, recalibrate investment theses in the biopharma sector, and force a national conversation on the meaning of accountability in an era of unprecedented scientific advancement and plummeting institutional trust. This briefing deconstructs the multifaceted crisis crystallized by the Tidmarsh resignation, analyzing the systemic pressures that precipitated it and charting the strategic implications for the future of drug regulation and public health.
The Anatomy of a Collapse: Pressure Points in the FDA’s Regulatory Framework
The resignation of a figure as central as the CDER Director does not happen in a vacuum. It is the culmination of mounting pressures that have strained the FDA’s framework for years. Understanding these dynamics is crucial to appreciating the magnitude of the current crisis and anticipating future regulatory behavior.
The Double-Edged Sword of Industry Funding
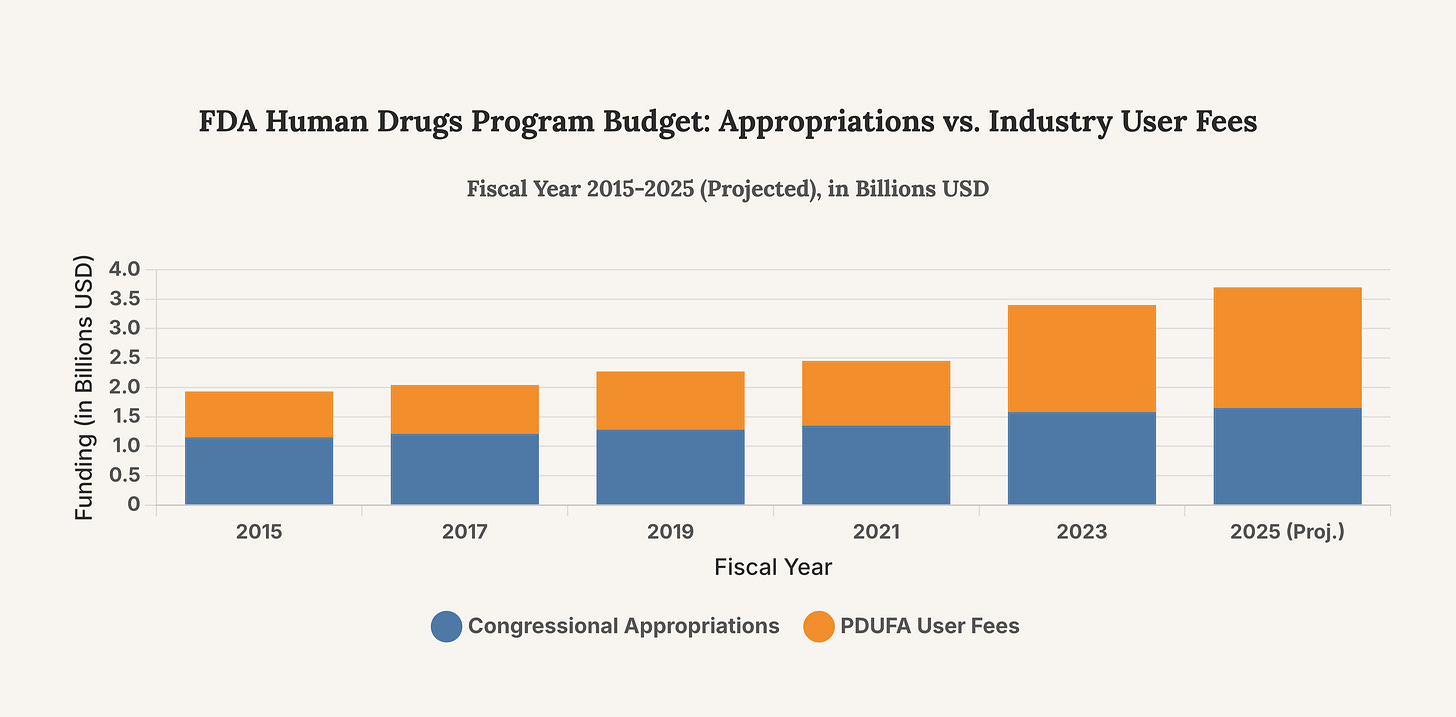
A core tension within the FDA is its increasing reliance on the very industry it regulates. The Prescription Drug User Fee Act (PDUFA), first passed in 1992, allows the FDA to collect fees from drug manufacturers to fund the new drug approval process. While this system was designed to expedite reviews and get crucial medicines to patients faster, it has created a dynamic of dependency. In fiscal year 2022, user fees accounted for 66% of the human drugs program budget. This financial reliance creates, at minimum, a perception of conflict, where the regulator’s financial health is tied to the volume and speed of applications from its clients—the pharmaceutical companies. Critics argue this has shifted the agency’s culture from a cautious gatekeeper to an accommodating partner, a concern that underpins many controversies, including the infamous approval of Vioxx in 1999, which was later withdrawn due to cardiovascular risks.
This chart illustrates the growing share of the FDA’s human drug program budget funded by industry user fees compared to congressional appropriations. The trend highlights the increasing financial reliance on regulated entities, a structural issue that forms the backdrop for concerns about regulatory independence.
The Accelerated Approval Pathway: A Pathway to Controversy
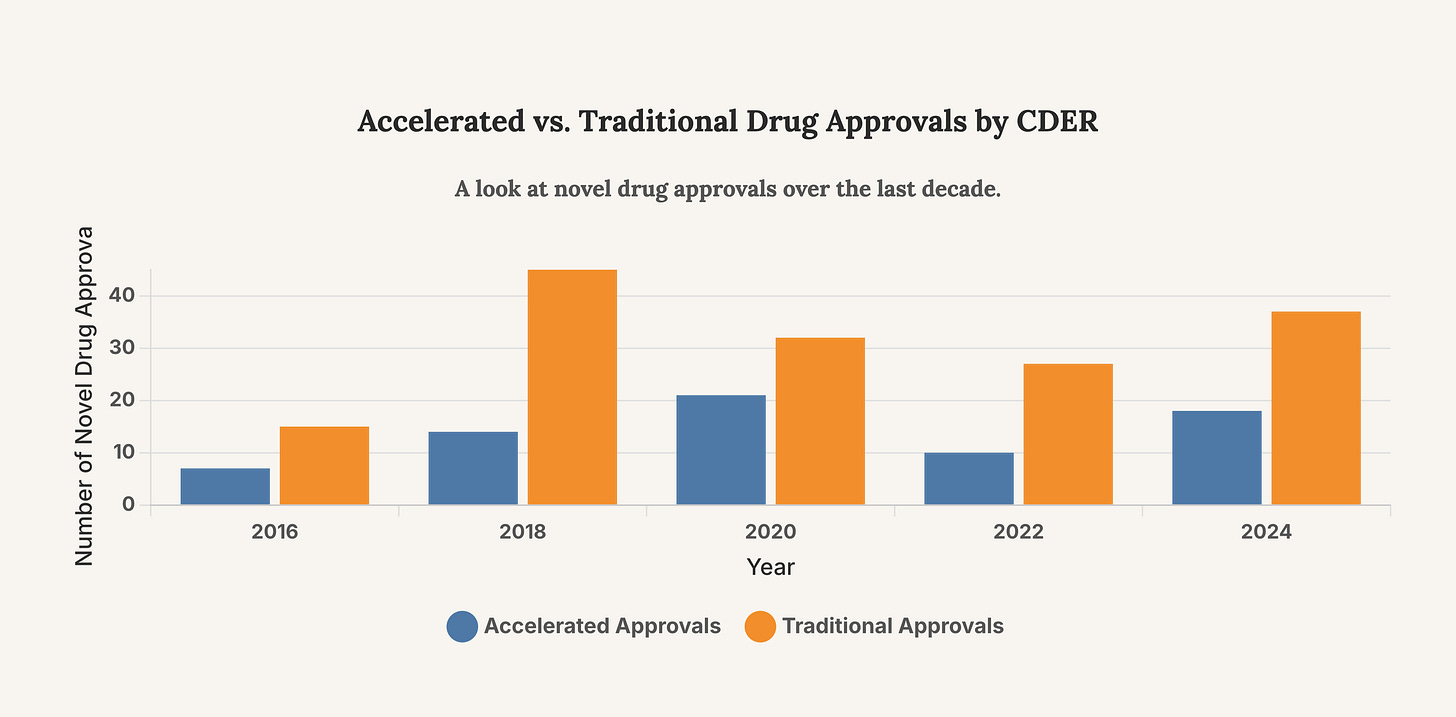
The controversy surrounding Dr. Tidmarsh is intertwined with critiques of the Accelerated Approval Program. Launched in 1992 to speed access to drugs for serious conditions, this pathway allows for approval based on surrogate endpoints—like tumor shrinkage—rather than proven clinical benefits, such as longer survival. However, the system is fraught with peril. A 2025 report from the HHS Inspector General highlighted significant concerns with the pathway, spurred by the contentious 2021 approval of the Alzheimer’s drug Aduhelm. The report found instances where the FDA failed to follow its own best practices, held undocumented meetings with sponsors, and suffered from a lack of oversight. These issues create a fertile ground for controversy, as drugs with uncertain benefits and high costs enter the market, placing immense pressure on regulators to justify their decisions.
The chart above shows the number of novel drugs approved via the accelerated pathway versus the traditional pathway. The significant number of accelerated approvals highlights the program’s importance and why its perceived integrity is critical to the FDA’s credibility.
The Crisis of Trust: A Failing Public Mandate
The ultimate currency of a public health institution is trust. The events surrounding Dr. Tidmarsh’s resignation are not merely procedural or ethical lapses; they are profound breaches of public trust that have been widening for years.
“Clear, consistent and open communication with the public and regulated industry...is a critical FDA function and essential for protecting and promoting the public health.” - Biotechnology Innovation Organization (BIO)
Eroding Public Confidence
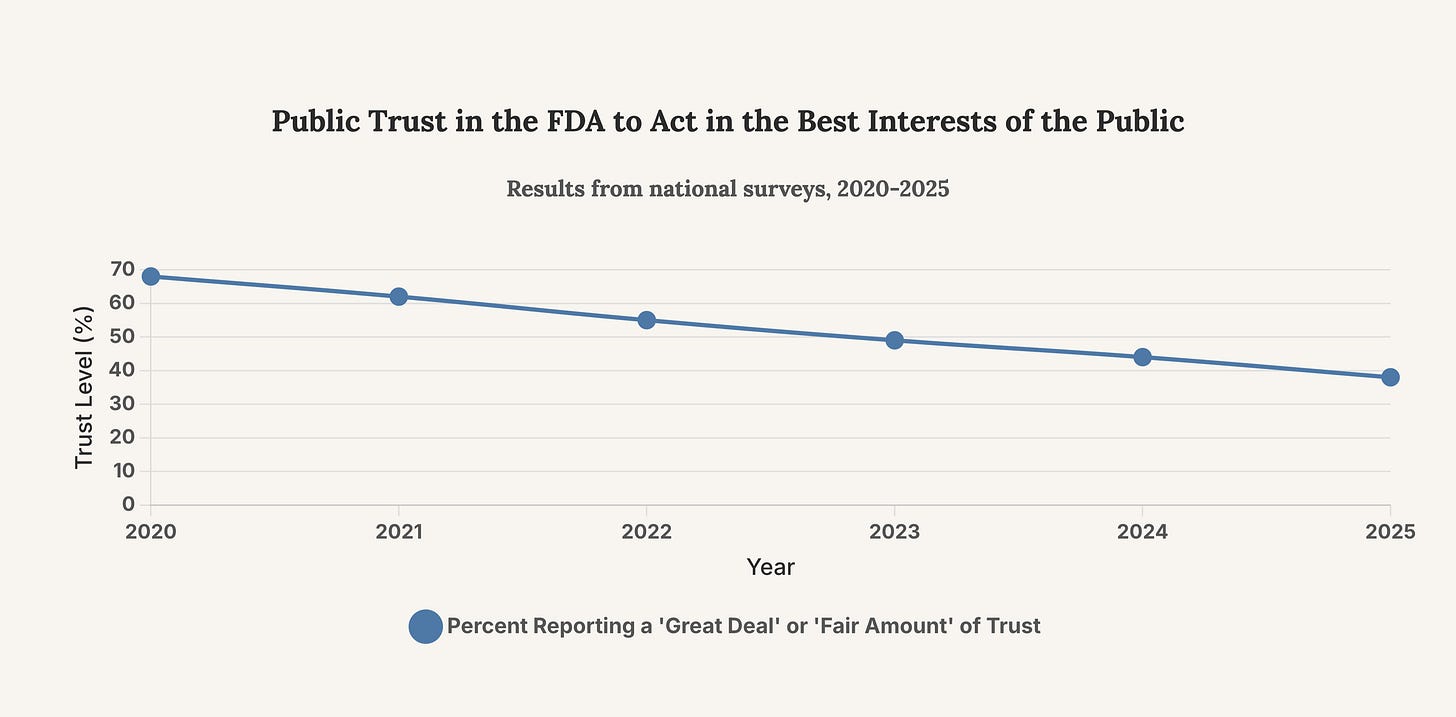
Recent survey data paints a grim picture of public confidence in the FDA. A May 2025 KFF poll found that fewer than half of Americans have confidence in the agency to carry out its core responsibilities. This erosion of trust is not abstract; it has tangible consequences for public health adherence, vaccine uptake, and the collective ability to respond to future health crises. The perception of political influence and a lack of transparency are key drivers of this decline. The Tidmarsh affair, with its allegations of personal vendettas influencing regulatory actions, pours fuel on this fire.
This chart tracks the decline in public trust in the FDA over the past five years. The steady downward trend underscores the urgency for reforms that can restore public confidence, a task made more difficult by high-profile scandals.
The Accountability Vacuum
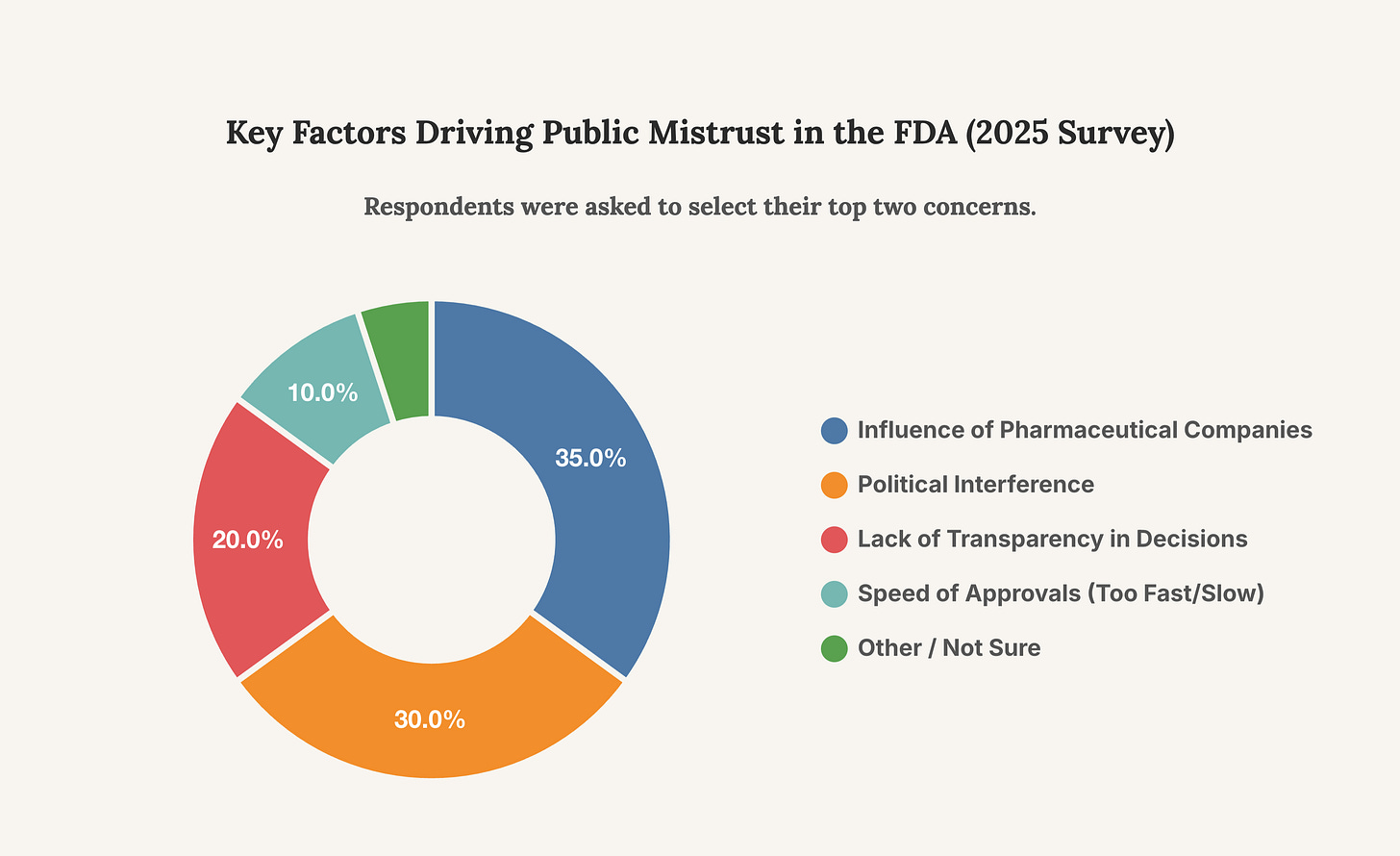
Accountability requires transparency, yet the FDA has often been criticized for its opacity. Efforts to increase transparency have been ongoing, with initiatives like publishing more data on Open.FDA.gov and, more recently in 2025, releasing past Complete Response Letters (CRLs). However, these steps are often overshadowed by controversies that suggest a reactive rather than proactive approach to accountability. The lawsuit against Dr. Tidmarsh, alleging he used his official position to settle personal scores, strikes at the very heart of impartial public service. Whether the allegations are proven true or not, their existence points to a system where personal conduct and regulatory power can become dangerously entangled, demanding more robust and transparent oversight mechanisms.
This chart breaks down the primary reasons cited for public mistrust in the FDA. The dominance of concerns about industry and political influence reveals where the agency’s credibility deficit is most acute.
Strategic Implications and Forward Trajectory
The fallout from the Tidmarsh resignation will be significant and far-reaching. Stakeholders across the healthcare, investment, and policy landscapes must prepare for a period of heightened scrutiny and potential reform.
For the Biopharma Industry: Navigating a New Regulatory Gauntlet
Companies must anticipate a more cautious and potentially slower FDA in the short to medium term. Expect increased documentation requirements for meetings, more stringent reviews of surrogate endpoint data in accelerated approval applications, and a lower tolerance for ambiguity in clinical trial results. Regulatory risk has now demonstrably increased. Investment in robust, unequivocal clinical data will be paramount. Companies with drugs in the accelerated approval pipeline, particularly those based on novel or less-validated surrogate endpoints, should brace for a higher bar for approval and prepare for more rigorous post-market study demands.
“There is growing public concern about the ability of the current drug safety system to prevent future Vioxx-like events.” - Institute of Medicine (now National Academy of Medicine) Report
For Investors: Recalibrating Risk and Opportunity
The investment thesis for biopharma must now more heavily weight regulatory risk. The binary outcome of an FDA decision has always been a key risk factor, but the integrity of the process itself is now in question. Valuations for companies heavily reliant on a single asset in the FDA pipeline may see increased volatility. Conversely, companies with strong clinical data, clear efficacy on primary endpoints, and a diversified portfolio may become more attractive safe havens. The market will likely reward transparency and punish any perception of cutting corners in clinical development.
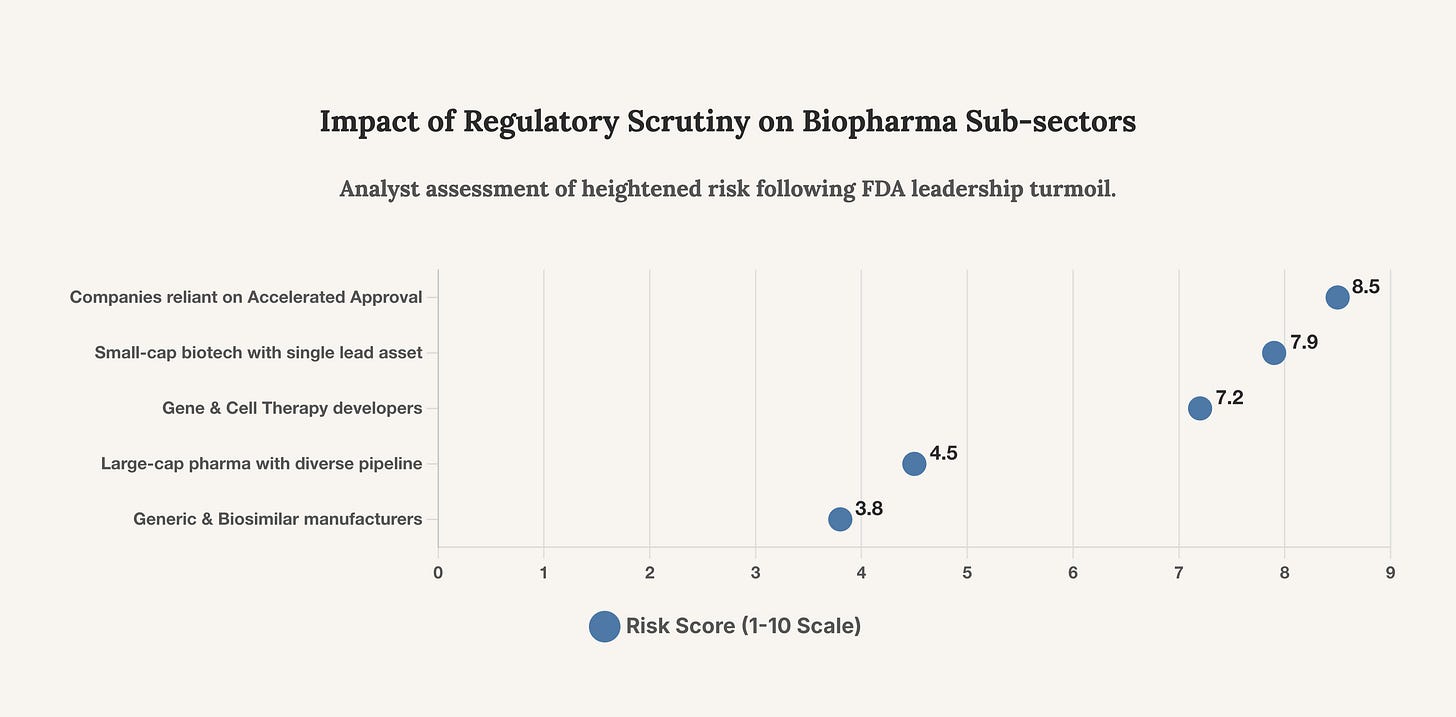
This chart provides a strategic assessment of which biopharma sub-sectors face the most significant increase in regulatory risk following the current FDA crisis. Investors must re-evaluate their exposure to the most vulnerable categories.
For Policymakers: The Imperative for Structural Reform
This crisis creates a political mandate for reform that cannot be ignored. Congress will likely intensify oversight of the FDA, with a particular focus on the PDUFA model and the accelerated approval pathway. Potential legislative actions could include new conflict-of-interest rules for senior FDA officials, stronger enforcement mechanisms for post-market studies, and even a re-evaluation of the user fee funding model. The FDA Reporting Transparency and Accountability Act, or similar legislation, may gain new momentum. The challenge will be to implement meaningful reforms that restore trust without stifling innovation or overburdening an already strained agency.
The Path Forward: Rebuilding the Gatekeeper’s Credibility
The resignation of George Tidmarsh is a symptom of a deeper malaise. The FDA stands at a precipice, its credibility compromised by structural dependencies, procedural inconsistencies, and a severe deficit of public trust. Restoring its status as an unimpeachable arbiter of science and safety is not merely an administrative challenge; it is a national security imperative. The path forward requires more than just a new director for CDER. It demands a fundamental recommitment to transparency, the fortification of firewalls between regulators and industry, and the establishment of unambiguous accountability for those entrusted with the nation’s health. Without these reforms, the cycle of controversy, scandal, and eroding trust will continue, leaving the public vulnerable and the future of medical innovation in jeopardy.
The true crisis is not the departure of one official, but the revelation of a system whose internal safeguards are failing under the immense weight of financial incentives and public expectation.